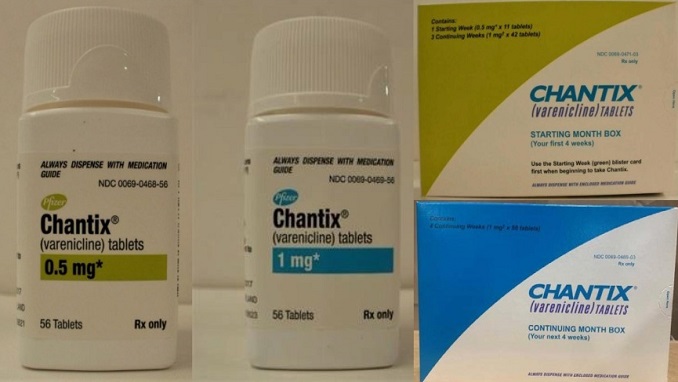
Pharma giant Pfizer has issued voluntary recall notice for all lots of its highly popular anti-smoking drug Chantix after analysis discovered it contains high levels of nitrosamine, which can increase the risk of cancer, USA Today reports.
The recall notice posted Thursday to the Food and Drug Administration’s (FDA) website concerns Chantix 0.5 mg and 1 mg tablets to the patient level due to the presence of nitrosamine at or above the FDA interim acceptable intake limit.
Chantix is an anti-smoking short term treatment that help patients quit smoking.
N-nitroso-varenicline is a nitrosamine, which are common in water and foods like cured and grilled meats, dairy products and vegetables, and everyone is exposed to it at some level, but it may increase the risk of cancer if above a certain limit.
The notice said that long term ingestion of nitrosamine can be associated with increased cancer risk in humans, but the company stressed there’s no immediate risk to patients taking the drug adding that benefits of stopping smoking outweigh the theoretical cancer risk from nitrosamine.
But since alternative suppliers have been approved in the US, Pfizer decided to undertake the precautionary measures.
FDA has updated the notice on Friday noting that patients should continue taking their drug until their doctors prescribe a different treatment or pharmacists provides them with a replacement.
The company’s latest recall concerns all lots of Chantix distributed from May 2019 to September to wholesalers and distributors in the US, US Virgin Islands, and Puerto Rico.
Pfizer recalled in August four additional lots of Chantix due to the impurity and 12 lots in July due to the presence of nitrosamine.



Be the first to comment